ARISTOTLE
Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation
ClinicalTrials.gov: NCT00412984
Patient Population:
- 18,201 patients with atrial fibrillation
- 3,417 US patients
- 18 years or older
- one or more risk factors for stroke
Trial Design: Double-blind, randomized, parallel assignment
Intervention: Apixaban in preventing stroke and systemic embolism in subjects with atrial fibrillation and risk factors for stroke.
Primary Endpoint: Composite of stroke and all-cause mortality
Sponsors: Bristol-Myers Squibb
US Economic Substudy
Principal Investigator:
Daniel B. Mark, MD
ARISTOTLE: U.S. ECONOMIC STUDY
Overview
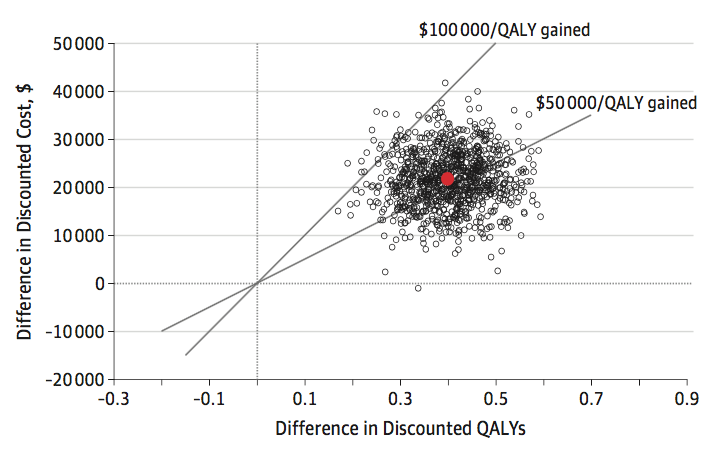
This patient-level analysis of the ARISTOTLE trial found no difference between treatments in health care costs (excluding anticoagulation costs) but significantly longer expected survival with apixaban therapy compared with warfarin therapy. Over a lifetime, apixaban treatment cost an additional $53 ,925 per quality-adjusted life-year gained.
Results
JAMA Cardiol. 2017 May;2(5):525-534
Importance:
The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial reported that apixaban therapy was superior to warfarin therapy in preventing stroke and all-cause death while causing significantly fewer major bleeds. To establish the value proposition of substituting apixiban therapy for warfarin therapy in patients with atrial fibrillation, we performed a cost-effectiveness analysis using patient-level data from the ARISTOTLE trial.
Objective:
To assess the cost and cost-effectiveness of apixaban therapy compared with warfarin therapy in patients with atrial fibrillation from the perspective of the US health care system.
Design, Setting, Participants:
This economic analysis uses patient-level resource use and clinical data collected in the ARISTOTLE trial, a multinational randomized clinical trial that observed 18 201 patients (3417 US patients) for a median of 1.8 years between 2006 and 2011.
Interventions:
Apixaban therapy vs warfarin therapy.
Main Outcomes and Measures:
Within-trial resource use and cost were compared between treatments, using externally derived US cost weights. Life expectancies for US patients were estimated according to their baseline risk and treatment using time-based and age-based survival models developed using the overall ARISTOTLE population. Quality-of-life adjustment factors were obtained from external sources. Cost-effectiveness (incremental cost per quality-adjusted life-year gained) was evaluated from a US perspective, and extensive sensitivity analyses were performed.
Results:
Of the 3417 US patients enrolled in ARISTOTLE, the mean (SD) age was 71 (10) years; 2329 (68.2%) were male and 3264 (95.5%) were white. After 2 years of anticoagulation therapy, health care costs (excluding the study drug) of patients treated with apixaban therapy and warfarin therapy were not statistically different (difference, -$60; 95% CI, -$2728 to $2608). Life expectancy, modeled from ARISTOTLE outcomes, was significantly longer with apixaban therapy vs warfarin therapy (7.94 vs 7.54 quality-adjusted life years). The incremental cost, including cost of anticoagulant and monitoring, of achieving these benefits was within accepted US norms ($53 925 per quality-adjusted life year, with 98% likelihood of meeting a $100 000 willingness-to-pay threshold). Results were generally consistent when model assumptions were varied, with lifetime cost-effectiveness most affected by the price of apixaban and the time horizon.
Conclusions and Relevance:
Apixaban therapy for ARISTOTLE-eligible patients with atrial fibrillation provides clinical benefits at an incremental cost that represents reasonable value for money judged using US benchmarks for cost-effectiveness.
ARISTOTLE Publications
Cowper PA, Sheng S, Lopes RD, Anstrom KJ, Stafford JA, Davidson-Ray L, Al-Khatib SM, Ansell J, Dorian P, Husted S, McMurray JJV, Steg PG, Alexander JH, Wallentin L, Granger CB, Mark DB.
JAMA Cardiol. 2017 May 1;2(5):525-534. doi: 10.1001/jamacardio.2017.0065.
- PMID: 28355434